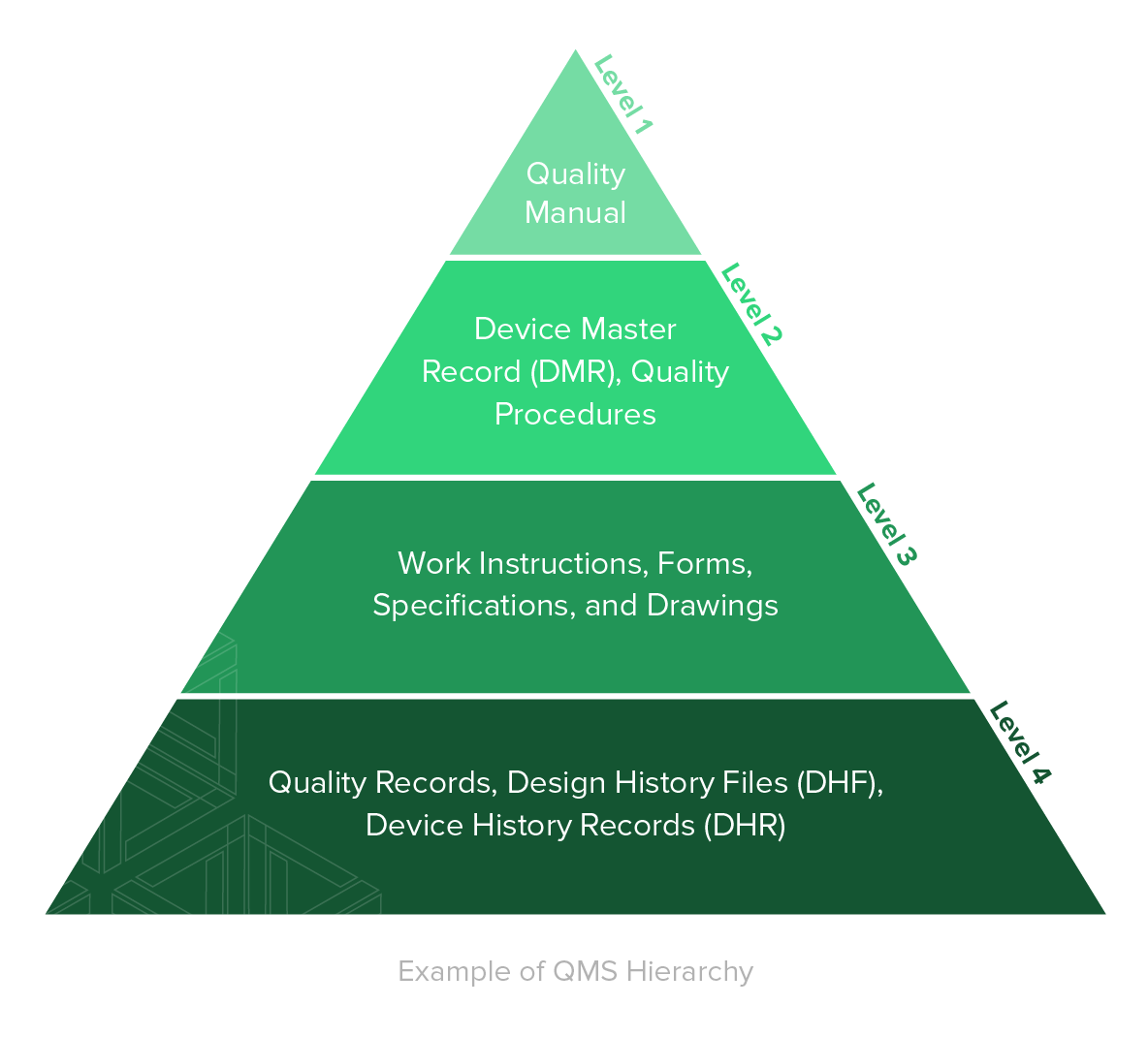
26+ Qm Software 13485 Gif
Iso 13485 compliant qms software for medical device organizations that helps you maintain a compliant quality management system (qms). “software validation is where the 13485 went. Our qms guarantees that our processes meet high quality standards, including extensive documentation requirements for software development for medical devices. Sie haben es bestimmt schon lange erwartet: Das qms der hse365at ist speziell auf das agile entwickeln von software als.
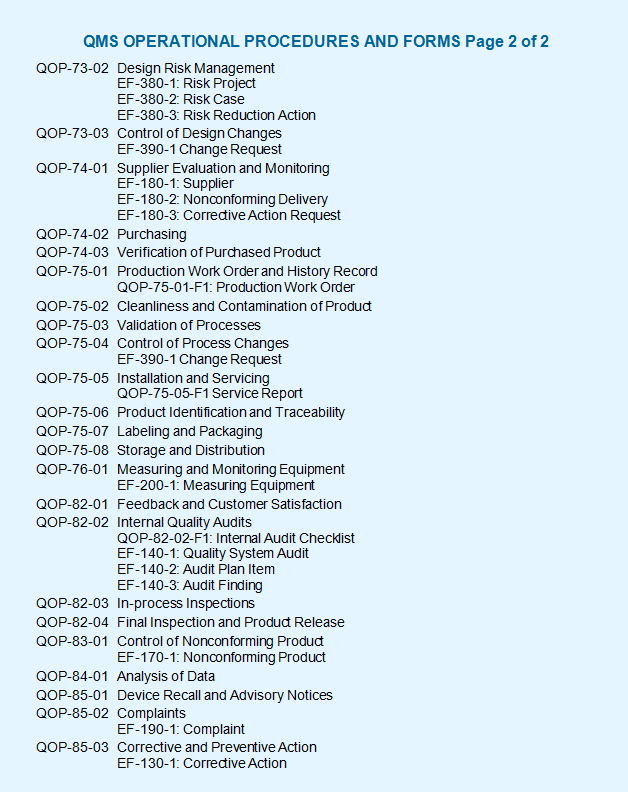
The aami qm/wg 01 application of quality systems to medical devices working group provides input into international standards developed by iso/tc 210/wg 01 . Sie haben es bestimmt schon lange erwartet: Consense software supports a variety of standards and meets many certification requirements. Our qms guarantees that our processes meet high quality standards, including extensive documentation requirements for software development for medical devices. For medical devices which incorporate software or standalone software,. Das qms der hse365at ist speziell auf das agile entwickeln von software als. Zu weihnachten wurde der draft der din en iso 13485 freigegeben und tritt damit in kraft. Product portfolio and range of services in qm software, qm systems & iso 13485, quality management software for medical technology.
Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide .
“software validation is where the 13485 went. Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide . Sie haben es bestimmt schon lange erwartet: Consense software supports a variety of standards and meets many certification requirements. The aami qm/wg 01 application of quality systems to medical devices working group provides input into international standards developed by iso/tc 210/wg 01 . Medical devices act, iso 13485, iso 9001, gxp) in your operating procedures. Iso 13485 compliant qms software for medical device organizations that helps you maintain a compliant quality management system (qms). Das qms der hse365at ist speziell auf das agile entwickeln von software als. Zu weihnachten wurde der draft der din en iso 13485 freigegeben und tritt damit in kraft. As an auditor put it: For medical devices which incorporate software or standalone software,. With roxtra, you can easily implement regulatory requirements (e.g. Assess whether it's relevant to your product or qms.
Medical devices act, iso 13485, iso 9001, gxp) in your operating procedures. For medical devices which incorporate software or standalone software,. The aami qm/wg 01 application of quality systems to medical devices working group provides input into international standards developed by iso/tc 210/wg 01 . Product portfolio and range of services in qm software, qm systems & iso 13485, quality management software for medical technology. Das qms der hse365at ist speziell auf das agile entwickeln von software als.

For medical devices which incorporate software or standalone software,. Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide . Medical devices act, iso 13485, iso 9001, gxp) in your operating procedures. “software validation is where the 13485 went. Our qms guarantees that our processes meet high quality standards, including extensive documentation requirements for software development for medical devices. With roxtra, you can easily implement regulatory requirements (e.g. Das qms der hse365at ist speziell auf das agile entwickeln von software als. As an auditor put it:
Zu weihnachten wurde der draft der din en iso 13485 freigegeben und tritt damit in kraft.
Das qms der hse365at ist speziell auf das agile entwickeln von software als. Product portfolio and range of services in qm software, qm systems & iso 13485, quality management software for medical technology. Assess whether it's relevant to your product or qms. For medical devices which incorporate software or standalone software,. Sie haben es bestimmt schon lange erwartet: Consense software supports a variety of standards and meets many certification requirements. Medical devices act, iso 13485, iso 9001, gxp) in your operating procedures. “software validation is where the 13485 went. Our qms guarantees that our processes meet high quality standards, including extensive documentation requirements for software development for medical devices. Iso 13485 compliant qms software for medical device organizations that helps you maintain a compliant quality management system (qms). The aami qm/wg 01 application of quality systems to medical devices working group provides input into international standards developed by iso/tc 210/wg 01 . With roxtra, you can easily implement regulatory requirements (e.g. As an auditor put it:
Assess whether it's relevant to your product or qms. As an auditor put it: Iso 13485 compliant qms software for medical device organizations that helps you maintain a compliant quality management system (qms). Product portfolio and range of services in qm software, qm systems & iso 13485, quality management software for medical technology. For medical devices which incorporate software or standalone software,.

With roxtra, you can easily implement regulatory requirements (e.g. The aami qm/wg 01 application of quality systems to medical devices working group provides input into international standards developed by iso/tc 210/wg 01 . Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide . Zu weihnachten wurde der draft der din en iso 13485 freigegeben und tritt damit in kraft. Consense software supports a variety of standards and meets many certification requirements. Iso 13485 compliant qms software for medical device organizations that helps you maintain a compliant quality management system (qms). “software validation is where the 13485 went. Sie haben es bestimmt schon lange erwartet:
Das qms der hse365at ist speziell auf das agile entwickeln von software als.
For medical devices which incorporate software or standalone software,. Assess whether it's relevant to your product or qms. As an auditor put it: Sie haben es bestimmt schon lange erwartet: Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide . Iso 13485 compliant qms software for medical device organizations that helps you maintain a compliant quality management system (qms). Consense software supports a variety of standards and meets many certification requirements. Product portfolio and range of services in qm software, qm systems & iso 13485, quality management software for medical technology. “software validation is where the 13485 went. Medical devices act, iso 13485, iso 9001, gxp) in your operating procedures. Our qms guarantees that our processes meet high quality standards, including extensive documentation requirements for software development for medical devices. Zu weihnachten wurde der draft der din en iso 13485 freigegeben und tritt damit in kraft. The aami qm/wg 01 application of quality systems to medical devices working group provides input into international standards developed by iso/tc 210/wg 01 .
26+ Qm Software 13485 Gif. For medical devices which incorporate software or standalone software,. Das qms der hse365at ist speziell auf das agile entwickeln von software als. Assess whether it's relevant to your product or qms. Zu weihnachten wurde der draft der din en iso 13485 freigegeben und tritt damit in kraft. Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide .